In a continuation in theme of my interest in indole synthesis, I found a fantastic mini-review on-line by Nadeesha Ranasinghe and Graham B. Jones at Northeastern University Dept. of Chemistry (Current Green Chemistry, available as an e-article and inprint 2015) on Flow and Microwave Assisted Synthesis of Medicinally Relevant Indoles…..this will be a must have for anyone interested in flow, microwave, or combination of the two as enabling technologies. And on a side note, Graham is a Liverpool football fan so that has to count for something.
Let me digress just a bit, because there have been so many advancements in the last 5 years, that one has to take a step back and see how it fits as we move away from traditional approaches to newer, current sustainable processes. We are at a pivotal point in our field* For a number of years our mechanistic understanding has been beyond our abilities to execute them, but now we are seeing the creativity amongst chemists at an accelerated rate with enabling technologies in microwave and flow chemistry methods. For the first time – we are reaching for the newest tools in the box to develop strategies that support our ideas and mechanistic creativity. Part of this growth is in line with automated purification and detection, as well as major advancements in X-ray and modeling technology – we are equipped to elegantly move forward over the hammer and tong approaches in the past.
Now that I got that out of the way, let’s go through the review – I will focus on flow and you can read some of the indole microwave details along with several installments on mw assisted indole syntheses at http://totallymicrowave.wordpress.com/, but the last section on the combination of the two will be covered briefly. The review does take a start with an historical perspective on the significance of indoles and their place in medicinal chemistry and drug discovery and changes gears to say that there has been focus on changing the way we perform our chemistry with newer strategies on-line. Jones describes two important points here: 1)that within enabling technologies is “enabling techniques” and that this is any technique that speeds up a synthetic transformation, facilitates easy work-up ad makes isolation/purification simple — all areas in alignment today….the only thing I would add is that it is sustainable; 2) changing the mindset of scale-up to “scale-out” as an underlining mantra to flow chemistry (i.e. we don’t have to change the chemical methodology from a traditional batch method if we start with a flow method, so we are simply using the chemistry in a larger capacity. OK, enough of that.
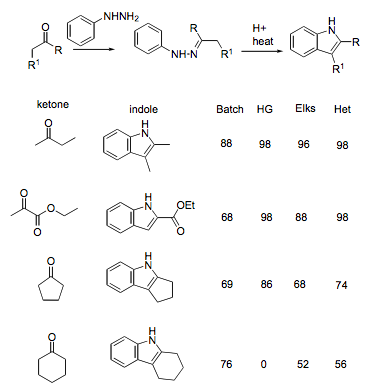
Early indole flow: the first pioneering effort to indole flow chemistry can be attributed to Paul Watts (Tetrahedron 2010) where he compared 3 methods to a conventional batch method.
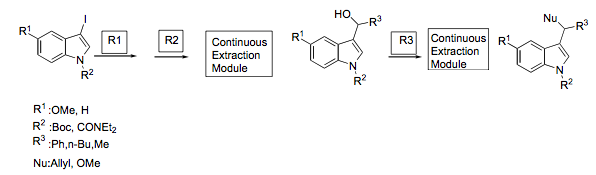
A second milestone came from the work done by O’Shea and Tricotet (Chem Eur Journal 2010) where they generated a series of 3-hydroxymethylindoles in an automated set-up with 2 flow steps and an in-line extraction module to trap and send the components through the remainder of the set-up, indicating that it is possible to perform multi-step flow chemistry in a straightforward way.
Moving into some of the more mainstream instrumentation available, Guillier reported a continuous flow hydrogenation in the formation of indole and 5-azaindole-2-carboxylates, a Reissert Indole construction, using an H-cube (ThalesNano) with pre-packed heterogenous catalyst cartridges with a flow of 1-3 ml//min and a temperature range of 20-100C ( J Flow Chem 2011).
A example of approaches can also be found in approaches to defined important indole intermediates. For examples, Kappe reported a flow route to 7-ethyltroptophol without the use of strong acids and bases — indicating we can move away from harsh conditions utilizing a flow approach (Org Process Res Dev 2013).
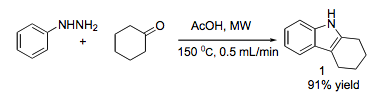
As I mentioned prior, I will highlight additional microwave approaches elsewhere, but it does serve to mention a couple of examples of the combination of mw and flow, or even areas where the transfer of technology is applicable in moving from mw to flow chemistry. For the first report on the combination of the two for indoles (MACOS) featured a simple flow cell made out of glass fitted with sand, attached to an HPLC pump for solvent flow and a back pressure regulator to produce 1g of the desired indole in 15-30 min @ 150C (91% isolated yield) (JOC 2005)……interesting that this combination pre-dates full use of flow methods.
The transfer of technology that is taking place can be found from microwave methods being used as a baseline for conditions to be used in continuous flow set-ups. Kappe published an approach to mimic microwave conditions in comparison using a stainless steel microreactor (spec, 350C and 200 bar capability) to produce 25 g of an advanced indole in 1 hour (EurJOC 2009).
The last example of an advancement of the combination is reported by Michael Organ (Chem Eur Journal 2008) where he illustrates the use of Metal-coated capillary tubes traversing the microwave cavity under flow control for sequential aryl amination/heck reactions in the construction of substituted indoles.
So hopefully this has helped with some perspective on the evolving field of continuous flow and combined approaches today. I thank Nadeesha and Graham for putting together a fabulous review for those of us interested in flow and indole chemistry. Happy Reading!




[…] minireview out of Northeaster University by Graham Jones and Nadeesha Ranasinghe that I posted on my flow chemistry resource blog — mainly because the journal article emphasis both continuous flow, microwave and the […]
LikeLike