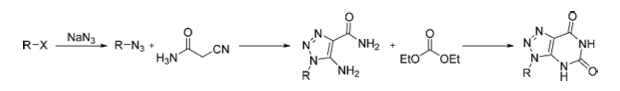
A recent example of reaction route scouting was published by a group at Northeastern University ( J Flow Chem 2014, Graham Jones et al) showing several critical factors going into the [3+2] azide cycloaddition chemistry in forming a desired triazole.
First thing – as they highlight, the triazole pharmacophore is a privileged structure indeed with a final portion of the paper indicating a successful Brilinta analog library. Depending on who you ask — metathesis or click chemistry with the most citations in recent memory, but one thing we call all agree, click chemistry utilization has made the triazole hot again, and this group chose a robust [3+2] cycloaddition of an azide with cyanoacetamide to place the amino-amide functionalities in place to form the requites triazolopyrimidone.
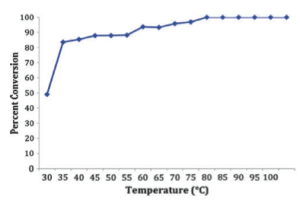
The group points out several details when using a methodology like this: dealing with hazardous organic azides, temperature for effective transformation and the number of equivalents compared with a traditional batch process. For a proof-of-concept and trial determination, the group utilized a Labtrix S1 system (Chemtrix) for the displacement of benzyl bromide with NaN3 in NMP to form smaller quantities of the small alkyl azide in a flow format while varying the temperature with flow rates of 0.5 to 25 microliters/min — so after a few minutes and less than 100C the final product is produced in high yield.
From here they were able to utilize the benzyl azide in a second flow reaction and cycloaddition with cyanoacetamide under mixing aq NaOH/NMP, followed by a study varying the amount of cyanoacetamide needed for full conversion (b, below). Lastly a combined 2-step one flow set of conditions were determined using benzyl bromide, NaN3 at 80C and 1 microliters/min followed by injection of NaOH/cyanoacetamide for a one-pot approach over the sequential process. A schematic of the flow is presented below, but because they wanted to make libraries, this 2-step one pot (a) approach allowed a library of triazoles to be made with commercial starting materials.
For purposes of the project, they reported the continued synthesis of Brilinta analogs following the triazole ring construction with a cyclocondensation to produce the requisite triazolopyrimidone, chlorination, and 2-independent heteroaromatic substitutions.
Although it can be imagined that the entire sequence could have been done in flow sequence, the group showed us that this technology can be used in a number of orders in a drug discovery approach — rather than simply thinking of this as a tool, I think this will help medicinal groups and process chemists have real discussions where these strategies can be optimally used for their work — not only can it be used in an analog campaign, but also for the larger production of a best compound intermediate (lets say compound 25 so that divergent analog approaches to high-speed analoging by independent team members take a chunk of the intermediate, thereby yielding more final, testable analogs…….thanks Northeastern (thanks Sara!). Happy Reading!