Look to join Flow Chemistry Society’s 4th International Conference on Flow Chemistry (Mumbai, Jan 21-22, 2016)– just take a look at the jam-packed list of presenters.


Rather than spend a chunk of time outlining developments of monoliths or scavengers, I decided that it would be better to push out some of the new ideas and exploration. We are aware that a number of groups are using Cu catalysts beds and cartridges, but a new publication uses a microchannel itself as the Cu source — followed closely behind the mixing of an azide and alkyne for the requisite step……love it. This process gives way to trying several types of catalysts to look at turnover numbers (TONs) and mechanisms themselves. For me, I would take the best and make a larger catalyst bed out of it so that scaling can be an option as well. Enough of that — collaborative groups out of Japan (Chemistry 2015) show us the way with their investigation of a Huisgen cycloaddition from the generation of 4 microchannel polymeric Cu membranes (will make you read into the formation — it looks elegant and formally pretty easy)– once formed azides and terminal acetylenes are flowed through the microchannels in a 3:1 mixture of Acetone:H2O at 50C in a residence time of 8 sec (some a few seconds longer, hahaha!– there is a difference in the the catalytic activity of each of the microchannel membranes but the reactivity is a significant enhancement over traditional catalysts and worth a note that flow can be a real progress to not only this chemistry but the concept in general.
Microchannel A or catalyst A if you prefer shows the best results from their study and also helps us recognize the differences in the Cu source and a preferred mechanism. Read further into what they propose as to why the process is so efficient — I have included a snapshot of their photos of the channel following the formation and post 24 hrs. Several options are available in the selection of solvent for the reaction as well. Further reading indicates that the reaction is available for more complexity and added functional groups adding to its’ utility.
I have included a table for their screening of the membrane A to show that this process is amenable to screening and library development – a criteria now maintained in the flow community for medicinal chemistry traction — hopefully there will be a number of people who take up the role of improving these possibilities rather than rely on commercial availability as a precursor. I see this as a natural trend to the development of these catalysts into a bed or bound so that it is amenable to standard medicinal chemistry processes (they did show some more advanced application to the development)……one can hope, right?
Enjoy the read!
Seems that photocatalysis has become front and center in 2015 with no signs of slowing down — much of this has been due to improved methodology and catalyst development – and finding profound chemistries to apply. For me, it has added new and exciting space to flow chemistry, basic research and drug discovery (well specialty chemicals as well). having said that, I still notice a hesitation in application simply because a large percentage of chemists haven’t spent a lot of time around these transformations — couple that with slow traction of chemists digging into flow methods. It is however, something the newer batch (haha!) of chemists are gravitating to and we will start to see a large number of dedicated publications in photo and improvements on flow metalations.
An interesting but not as recent as you would think comes out of the MIT flow group in 2012-2013 utilizing visible light photoredox catalysis in flow. As is with the case of general flow synthesis (over a catalyst bed or at high temp and pressure) the larger surface to volume ratio allows for more control of temp and reaction times — and in this case more efficient irradiation. Within the paper, both oxidative and reductive provide some extremely powerful synthetic manipulations under these conditions and provide a nice framework to advance some of the initial ideas. Overcoming traditional batch light penetration with simple PFA tubing suggests that scale-up of once difficult batch processes will be easily overcome.
Take a look through some schemes that we would normally think of in medicinal chemistry efforts — Easy to perform and powerful — too bad I missed the boat on this one. Enjoy the read — it is thought provoking.
The second area I have been keeping an ear to the ground comes in the newer development in metalation — usually in regard to arenes or heteroarenes and the requisite quench. Expansion out of nBuLi to other Lithiates or Mg, Zn and other possibilities certainly seems to pave a way for new reactions with commercial reactors or at least some ideas on how to apply to your own reactor. Having discussions with fellow chemists on their approach to activate arenes with subsequent quenches remains high on the list of powerful methodologies so improvements that traditional batch chemists can sink their teeth in flow will go a long way in pushing people over to new ways to do their work. A recent publication (Chemical Science, 2015) out of Knochel’s lab provides some insight into a modified flow/batch scheme with Cy2NLi activation in flow followed by a variety of quenches — great to see a commercially available reagent applied with success so that we understand what we can and can’t do….really valuable stuff. Looks to me that we can start to design all sorts of reactions — both high temp/high pressure or simplified kinetic processes over cooling liter reactions to -78C.
Happy Reading!
Often times I hear frustration from the pharma community on the types of examples that are used to show applicability of instrumentation in continuous flow synthesis. Perhaps it is simply too time consuming to sieve through the literature to find a match to the project at hand….and flow examples are still in their infancy compared with examples of microwave examples — we need a great book of examples broken down by transformation (ala, Larock or by formation of privileged structures). Believe me, I am in front of this everyday and it is a job to find something that sparks the interest of many, but upsetting that traction has not taken root in our synthetic communities as well as it should for this technology.
Ha — now that’s better… a recent article had me thinking where many would fold up shop, this group dissected some of the usual development it takes to improve a reaction in flow: solubility, treatment of dangerous reagents, flow of more than one component in a reaction and the applicability of the research. A recent publication on the synthesis of hydantoins provided an excellent look at some of the issues, but also a concise plan for developing methods using flow designs:
Julia Monteiro, Bartholomäus Piebera Arlene Corrêa, Oliver Kappe Synlett 2015
https://www.thieme-connect.de/DOI/DOI?10.1055/s-0035-1560317
Below I have indicated the scheme followed by a table of yields under optimized conditions:
What you don’t see is how they get there and what issues are present at the starting point (makes for great problem solving): The first that becomes apparent is the use of KCN as a dangerous reagent and how things can play out on scaling, the second is how to mix organic and aqueous based reagents together without precipitation or clogging at high temp (certainly sounds at first that this would not be something to start with), and lastly would the proper flow of the reactants provide addition of the CN and ammonia groups followed by CO reaction and rearrangement in enough time to provide a useful set of transformations. Of course the answer to all if these is yes — each works well, but how about the perspective in thinking……KCN at high temp and some pressure –well the get thing about continuous flow is that there is no head-space and at high temp no room for the generation of dangerous gas reactants [it is a huge benefit that is not talked about enough]. Secondly, mixing of a biphasic solution — this is a bit challenging and an interesting read — you have to time the pumping of reagents and the mixing isn’t intuitive but something that can be achieved by telescoping with several solvents to maintain a laminar (as close to as needed) flow for the reaction and once proper mixing is achieved, the adequate Tres can be achieved with 2 separate pumps.
The group went on to show a match comparison with a microwave batch version resulting in lower conversion to the product for many of the initial reasons talked about. With that in hand, they went on to show that a number of changes in the starting aldehydes and ketones provided excellent yields of a variety of hydantoins….so you can see this should be a great concept generator of similar scaffolds that can be constructed in a similar manner.
Happy Reading!
While I spend most of my time on flow hydrogenation, and the transfer of this technology from discovery or feasibility to scale, there has been a lot of discussion (and movement to go along with it) on photo flow chemistry and flow electrochem….and it has taken a number of traditional batch organic chemist a minute to readjust their thinking — we would normally not consider photochem on a large batch scale in a number of industries but that is not the case today — and we are re-educating ourselves to these potential strategies — and now the same thing can be said of electrochem…and the list is going into several areas we have long forgotten (flash vacuum pyrolysis for example). Great to see technology contributing in areas where we can realize the potential as we have seen with hydrogenation applications, C-C coupling and catalyst development.
Rather than several examples of each, I highlight a 2012 review in photo flow that is easily found. Take a look through and follow through with some of the more recent efforts — I would be interested to hear back on whether there is a stronger interest in photo applications or electro AND whether any particular types of chemistry would easily scale under continuous conditions — MY IDEA is to post a discussion on people’s thoughts and any particular highlights from experience.
Enjoy!
Last chance to sign up for 2-day flow chemistry course in Nice — June 2,3 2015
There is a new short course on flow chemistry being offered by Scientific Update in Nice France (June 2-3 2015) with Oliver Kappe and Will Watson as instructors…..should be a great course with Oliver as a leader in the field of new flow chemistry information and current state-of-the-art techniques. I have included the information on the side – In the News (can also be found on the Kappe Group site). Anyone in the field should plan to visit and join the group in France for the latest education in flow chemistry….looks good to me!
I have recently had the opportunity to meet and work with Dr. Frank Gupton. He has made a career in the chemical development of drug candidates at more than one pharma entity (Hoechst and BI to name a couple)….and has taken his talents back to academia at VCU as the department chair of the Chemical and Life Science Engineering department…..quite a facility. Although industry has made an impression in his career, he is making a tremendous impact in continuous methodologies in manufacturing and drug discovery strategies using flow chemistry — and in several areas: catalyst development, API production, impactful methodology including hydrogenation, C-C coupling catalyst development, C-H activation and general approaches to specific targets. This practical paradigm is leading the way with industrial chemists building back into technology shifts across the pharma industry. One recent endeavor I have had the opportunity to join — Center for Rational Catalyst Design – a consortium with the University of South Carolina (with John Regalbuto) and some of the leading catalyst researchers in the country — all with a focus on development and research directed to new catalysts and basic research designed to impact all chemical industries. Anyone in pharma or metal catalyst design should take the time to contact Frank and the CeRCAS for an opportunity to stay in front of the newest developments in ongoing chemical catalyst design and flow chemical methods.
A recent example can be found in a continuous flow application in the synthesis of telmisartan. That said you should stay tuned some some of the newer work in catalyst and method development work……I will keep you posted.
Having been in the pharma industry most of my career I am a bit biased and probably to a fault. I do see a number of like-minded people within the industry without an ear to ground on what other industries have offered us over the years in there own bubbles. Let me say that what people do in the petro, catalyst and fine chemical industries will vary vastly to what is important in the pharma community, both on methods and scale. And by and large, never the twain do they cross — almost to an alarming amount.
One area I think the medicinal and process chemists in pharma can take a step back and think about would be in the area of high temperature and pressure flow chemistry — the FDA has open arms out on the manufacturing side, so why not get the stakeholders together in discovery and see where the limitations and opportunities exist. My phone has been ringing on where the possibilities might be — let’s take a step back — the IP space has kept the Pharma and Biotech armed against each other over the last 50 years — and although the dynamics are complex, we have been limited by temp and pressure from traditional roots……think about the advances in our recent past – -catalyst development for C-C and C-N formation, CH activation, microwave and flow and this has had a way of lowering energy barrier to doing new small molecule synthesis. I would contend however that we have been limited in temperature to about 250C and 25-30 bar of pressure even including microwave. Why not expand this capability to 500 or 1000C and extend to pressures of 350 bar — just look at the graph below (to include supercritical fluid possibilities). The reason I frown and hold my head low is that I have seen so many pharma companies crippled by the same compounds — how many times am I going to come across the same chemical core — any new cores out there people?
Now that I have the subject set, I have included some homework for all — there are 50-60 current publications showing that it is possible to utilize higher temps and pressures to generate new chemical matter and new strategies — no more excuses that my compound won’t stand up to 350C — think about the residence time as a driving force in the strategy. Below you will see 3-4 approaches to why drug discovery chemists should take this seriously.
In the following slide, Oliver Kappe shows a baseline approach in moving from microwave to CF technology — in doing so, not only do we see how a process intensification works, it also speaks to the way people are thinking in med chem — “I have a reaction at reflux for >6hr and the microwave at 200C brings it down to 10-20 min, then the residence time in flow should be on that order” — and if the reactor allows for a combination of high temp and pressure with a suitable pump, we can access multiple types of reaction space and more importantly IP space. A comparison on three examples start to flesh out that we should include this thinking in our strategies.
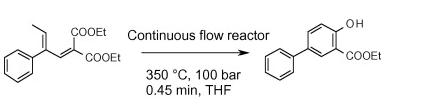
Once you get past the inhibition of working above 250C or higher pressures — or both, a different thinking starts to take over….I can use solvents that I can’t apply in the microwave…..I can use solvents that can’t be used in batch at high temps — keep in mind that flow doesn’t have any headspace so THF at 300C is safe. An example below really scratches the surface of how this thinking can be applied — simplifying an old reaction [Gould-Jacobs quinoline synthesis] provides a process to access the desired heterocycle in fewer steps, eliminates the use of high boiling solvents and opens the door previously undescribed chemical space. The scheme below was described in Lengyel L., Nagy T. Zs., Sipos G., Jones R., Dormán Gy., Ürge L., Darvas F., Tetrahedron Lett., 2012; 53; 738-743. At an empirical stage at this point is fortuitous…..this will get mapped out at some point and predictive, but now is an excellent time to include this in the toolbox along with the photochemical and process that haven’t received their due. Added value to this approach can be found in Synthesis of Condensed Heterocycles by the Gould-Jacobs Reaction (OPRD 2015).
Other examples of opportunity — a number of these unimolecular reactions have application but an unmet need in the lab — just waiting for a young enthusiastic chemist ready to make a name for him/herself.

Have fun with the reading — embrace it….it’s why we got into this profession.
Enjoying the efforts of of Christopher Hone and his jflowchemistry blog. His latest article on catalyst, organic and liquid phase models for flow reaction chemistry was intriguing to say the least. There is a lot of interest in the ability to mix organic and aqueous phases – and then add reactive gas into the mix as it were. His description of Yap and co-workers at the National University of Singapore indicates that feasibility studies are ongoing to have a cleaner picture of what is controlling this sort of reaction (mass flow, heat transfer). What I enjoyed most is the subtleties of moving from a fixed bed or homogeneous catalysis into a design with the catalyst immobilized in the aqueous phase and work out the parameters — mixing, organic phase wetting, and finally introduction of gas. With the improvement and advancement of catalyst design — nanocatalyst — we may well see a number of new things being applied to flow design in the near future.
Nice job Chris — keep em coming! Happy reading to the audience!